What are biopharmaceutical medicines?
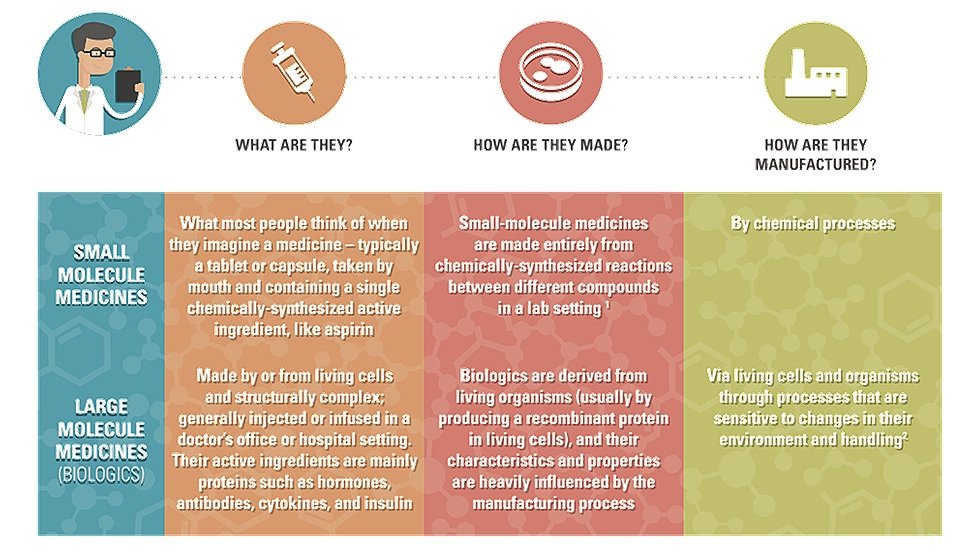
What is the first thing that comes to mind when you think of the word “medicine”? For many people, you may think of drugs such as Advil (ibuprofen) or Tylenol (acetaminophen). These types of medicines are small-molecule pharmaceutical drugs that are manufactured through chemical reactions. However, you may also think of other medicines, such as vaccines or antibodies, which are classified as biopharmaceutical drugs. Biopharmaceuticals (also known as biologics) are complex, larger-molecule medicines that are produced and derived from living organisms. Due to their molecular complexity, biologics are difficult to replicate. This means that only a small number of companies are able to manufacture each biopharmaceutical product and make the medicines available to patients.
Examples of biopharmaceutical medicines:
There are many types of biopharmaceutical medicines used to help prevent, treat, and cure a large number of illnesses and diseases. For instance, biologics can be used to treat cancers, cardiological diseases, respiratory illnesses, gastrointestinal issues, and many more diseases across a variety of medicinal fields. Generally, a biopharmaceutical medicine can be classified under a broader category of biologics, depending on the type of product and how it is produced. Below are some examples of types of biopharmaceutical products that currently exist:
1. Vaccines
Vaccines are biopharmaceutical products that help teach our immune systems how to fight an infection so that our bodies are prepared for future infections. They are produced using cells or viruses, depending on the type of vaccine being manufactured. There are a few different types of vaccines, such as: 1) live-attenuated vaccines, 2) inactivated vaccines, 3) subunit vaccines, 4) toxoid vaccines, 5) messenger RNA (mRNA) vaccines, and 6) viral vector vaccines.
You can learn more about these different types of vaccines at the following link: https://www.pfizer.com/news/articles/understanding_six_types_of_vaccine_technologies.
Also check out our article about the COVID-19 mRNA vaccine and how it works to prevent future infections: https://www.projectbrainlight.org/blog/covid-19-mrna-vaccine.
2. Therapeutic proteins
Therapeutic proteins are medicines that target proteins in order to treat diseases. Just like vaccines, there are many different types of therapeutic proteins that work in different ways, depending on the disease they are trying to treat or cure. Therapeutic protein treatments can have their effect by: 1) replacing abnormal proteins in the body that contribute to a disease, 2) enhancing the actions of proteins that already exist in our bodies, 3) blocking the action of a molecule or organism in the body, or 4) introducing new proteins into the body.
One example of a therapeutic protein that you may be familiar with are monoclonal antibodies. Monoclonal antibodies are typically used to block the actions of molecules (such as viruses) or organisms (such as bacteria) in the body that are contributing to a disease. Learn more about monoclonal antibodies at the following link: https://www.youtube.com/watch?v=M3zllm8QbCM.
3. Cell and gene therapies
Cell and gene therapies work on a molecular level to help prevent, treat, and cure disease. Cell therapies typically take effect by altering cells that contribute to a disease and then introducing those altered cells in the body, or by introducing new cells into the body that can help combat the disease. Gene therapies generally work by changing, inactivating, or inserting genes into specific cells, then introducing those cells into a patient’s body.
One example of a cell therapy is CAR T-cell therapy used to treat lymphoma. T-cells are a type of white blood cell that have an important role in our immune response to infection. Scientists can take T-cells from our body, add something called a chimeric antigen receptor (or CAR) to them, and then infuse the modified T-cells back into our bodies. The CAR T-cells are better able to recognize and fight off cancer cells than the original, unmodified T-cells in our body. Learn more about the therapy at the following link: https://www.yescarta.com/fl/treatment-process/.
Cell Therapies
Gene Therapies
How are biopharmaceutical products developed?
There are many stages involved in developing and manufacturing biopharmaceutical products. The entire process can take up to 10 years from development stages to the final launch and distribution of the product. This process can happen more quickly when there is an imminent need for a new biologic product, such as with the COVID-19 vaccine. It is important to note that when development is expedited due to public health crises, the biopharmaceutical product is still regulated to ensure that it is of high quality, effective, and safe. Overall, each step of the development process is necessary in order to determine the most effective, efficient, and safe way to produce a medicine that targets the intended disease.
1. First, scientists decide on the type of drug that they want to develop. Many of the biological processes that underlie medical problems have been well-researched. Therefore, scientists can design their biologic to target one of these known processes.
For instance, cancers are caused by mutations of the cells in our body. One way to treat cancers is to develop biologic cell or gene therapies that try to modify the genes within cancer cells that make them cancerous, to introduce new cells into our bodies to help fight off the cancerous cells, or to help our innate immune cells to better recognize and fight off the cancer cells. On the other hand, vaccines are a more effective biologic medicine for fighting off viral infections because they teach our bodies to recognize the viral molecules and destroy them before the virus has a chance to spread within the body.
The mechanism of how cancer causes our bodies to become sick (mutated cells that spread throughout the body) and how viral infections cause our bodies to become sick (killing healthy cells in the body) are different, which means that the way we treat, prevent, and cure these diseases will require unique biologic approaches.
2. Once the biologic drug is designed, preclinical studies are conducted to test the product in vivo or in vitro. These studies are not performed on humans, but are necessary to inform us about the safety and efficacy of the product before moving on to human trials.
In vitro (Latin for “within the glass”) studies are performed in a dish and typically involve living cells. These studies are performed to determine the properties of a drug and how it works at a cellular level in the body.
In vivo (Latin for “within the living”) studies are performed in a living organism and typically involve animal models. These studies are performed to determine the properties of a drug and how it works at the whole-body level, and help us determine the safe dosage of a drug that can be later administered to humans in clinical trials. In vivo studies of therapeutic drugs are required by the U.S. Federal Drug Administration (FDA) in order to ensure that the drug is safe and effective before it is tested in humans.
Animal models are currently the only method that can tell us about how a drug works within a full living organism. Therefore, researchers and veterinarians ensure that the animals involved in preclinical testing are well cared for and treated humanely. You can learn more about the importance of animal models in preclinical research at the following link: https://www.psbr.org/animal-research-2/general-overview.
Also check out our article about the benefits of tickling rats in the lab: https://www.projectbrainlight.org/blog/rat-tickling.
3. After the biologic drug is determined to be effective and safe in preclinical studies, clinical trial testing in humans can begin. Clinical trials are performed in order to identify if the drug has its intended effects in humans, if it is safe, and if it produces any side effects. We can then determine the most appropriate dosage of the medicine that produces the desired effect with the least amount of side effects.
Learn more about clinical trials at the following link: https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies
4. Once the FDA approves the biopharmaceutical product to be used in the intended patient population, the drug can be manufactured on a large-scale and distributed to patients. Some biologics must be administered by a physician in a hospital setting, whereas others can be administered in a pharmacy setting or even at home. The specific shipping, storage, and administration requirements can vary depending on the type of biopharmaceutical and the type of medical problem it is addressing.
Overall, biopharmaceutical products can be used to prevent, treat, and cure many types of diseases across a variety of medical fields. For example, vaccines are important for preventing viral infections and for helping our bodies know how to fight off the infection if it does occur. Additionally, CAR T-cell therapy helps fight and destroy cancer cells in our body by modifying our T-cells to better recognize the cancer cells. Throughout the process of developing a biopharmaceutical product, many government organizations, such as the Food and Drug Administration (FDA), and private organizations, such as the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL), aid in supporting the innovation of new biologic products and ensuring that high standards are met at all stages. With the invention of several new cutting-edge biomedical technologies within the last decade, the development of new biopharmaceutical therapies usings these tools offers a promising future for illnesses and conditions that currently lack other treatment options.
References and Additional Resources:
Here are some links to the resources listed in this article, as well as other resources that may help you better understand biopharmaceuticals.
What are biopharmaceuticals: https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/V-Z/what-makes-biologics-different.pdf
Vaccines
Video about vaccines: https://www.youtube.com/watch?v=2t_mQwTY4WQ
Learn more about the different types of vaccines: https://www.pfizer.com/news/articles/understanding_six_types_of_vaccine_technologies
Therapeutic proteins
Video about therapeutic proteins: https://www.youtube.com/watch?v=a6Rqzq_cJaY
Learn about therapeutic proteins: https://www.astrazeneca.com/r-d/next-generation-therapeutics/therapeutic-proteins.html
Learn about monoclonal antibodies: https://www.youtube.com/watch?v=M3zllm8QbCM
Cell and gene therapies
Learn about cell and gene therapies: https://asgct.org/education/more-resources/gene-and-cell-therapy-faqs
Learn about CAR T-cell therapy: https://www.yescarta.com/fl/treatment-process/
Video about cell therapies: https://www.youtube.com/watch?v=lEJXzTULVkI
Video about gene therapies: https://www.youtube.com/watch?v=hn-VCthxdPc
Miscellaneous links about biopharmaceutical development
The importance of animal models in research: https://www.psbr.org/animal-research-2/general-overview
Clinical trials: https://www.nia.nih.gov/health/what-are-clinical-trials-and-studies
Role of the Food and Drug Administration (FDA) in biologics: https://www.fda.gov/vaccines-blood-biologics/science-research-biologics
Learn about the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL): https://niimbl.force.com/s/about-niimbl
Full STEAM Ahead articles that are related to this topic:
Article about the COVID-19 mRNA vaccine and how it works to prevent future infections: https://www.projectbrainlight.org/blog/covid-19-mrna-vaccine
Article about the benefits of tickling rats in the lab: https://www.projectbrainlight.org/blog/rat-tickling